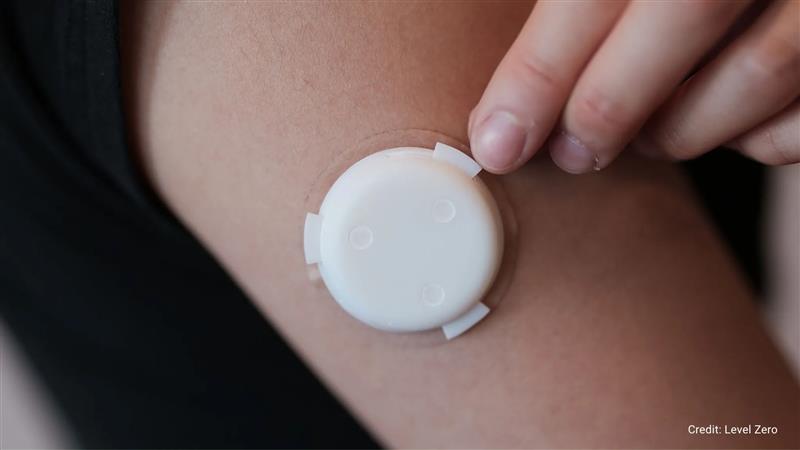
Ula Rustamova and Irene Jia, co-founders of the startup Level Zero Health, are developing a continuous hormone monitoring technology that focuses on tracking testosterone levels and in vitro fertilization (IVF) needs.
Much like continuous glucose monitors (CGMs) that reformed diabetes management, Level Zero’s new hormone monitoring device creates a similar reform in hormone health.
Although is it still in the early stages of development, it seems this continuous hormonal monitoring device has potential.
“We know now how much they regulate in terms of your day-to-day life,” said the startup’s CEO Rustamova in an interview with TechCrunch, explaining the importance of hormones and the way it affects different parts of the body, stating, “All of that is regulated by your hormones.”
Aptamers-Based Monitoring Device
Level Zero’s device would implement a sensor-based-on aptamers, smaller DNA molecules that are bound to respective hormones. Aptamers can detect hormone density via electrochemical and optical changes.
Chief Technology Officer (CTO) Jia defines these as “single-stranded DNA molecules that bind to target hormones,” thus enabling the sensor to pick up on real-time hormone levels.
The startup is developing its device based on the needles of the CGMs, which are already FDA approved, sampling interstitial fluid. This fluid is usually found around the cells, including progesterone, estrogen, cortisol, and testosterone.
Since the science related to continuous hormone monitoring is still developing, establishing such technology would be difficult. Researchers are however hopeful, because new research has demonstrated that aptamers are effective at detecting hormones such as progesterone.
Experts’ Support and Partnerships
The hormone monitoring device has already hit a major milestone in detecting progesterone at clinical levels in the interstitial fluid with its prototype and has locked in partnerships with US IVF clinics, with clinical studies to be conducted in 2025. To that, it sets a timeline for manufacturing engineering next year, while full clinical trials and FDA approvals are expected in 2026.
“We’ve spent a lot of time with fertility, perimenopause, and PCOS experts to make sure our data’s relevant,” said Jia. “That’s why experts from places like Harvard and Mount Sinai have joined our advisory team.”
If Level Zero’s technology works, it will reform the future of hormone monitoring, providing patients with more reliable, real-time insights into their health. It has also gained the interest and support of experts in the medical field.
It is worth noting that the device will be available for consumers through healthcare providers. While home kits, which measure hormones excreted in urine or saliva, often give unreliable results, this device would provide a much more accurate reading-as would be the case with blood tests, but with the advantage of it being continuous.
Inside Telecom provides you with an extensive list of content covering all aspects of the Tech industry. Keep an eye on our Medtech section to stay informed and updated with our daily articles.